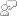SCALE GUARD

Water
Water resources are potentially useful to humans. Water usages include agricultural, industrial, household, recreational and environmental activities. Virtually all of these human uses require fresh water.
97% of water on the Earth is salt water, leaving only 3% as fresh water of which slightly over two thirds is frozen in glaciers and polar ice caps. The remaining unfrozen fresh water is mainly found as ground water, with only a small fraction present above ground or in the air.
Fresh water is a renewable resource, yet the world's supply of clean, fresh water is steadily decreasing. Water demand already exceeds supply in many parts of the world and as the world population continues to rise, so too does the water demand.
Hard water
Hard water has high mineral content (in contrast with soft water). Hard water minerals primarily consist of calcium (Ca2+), and magnesium (Mg2+) metal cations, and sometimes other dissolved compounds such as bicarbonates and sulfates. Calcium usually enters the water as either calcium carbonate (CaCO3), in the form of limestone and chalk, or calcium sulfate (CaSO4), in the form of other mineral deposits. The predominant source of magnesium is dolomite (CaMg(CO3)2). Hard water is generally not harmful to one's health.
Measurement of hardness
The total water 'hardness' (including both Ca2+ and Mg2+ ions) is read as parts per million (ppm) or weight/volume (mg/L) of calcium carbonate (CaCO3) in the water.
Scale Formation
Hard water causes scaling, which is the left-over mineral deposits of Calcium and magnesium salts , also referred as hardness salts
Scale Formation depend two factors: Temperature & pH
As the temperature of water in cooling circuit increases, it causes scale formation on heat transfer surfaces.
As the temperature of water in cooling circuit increases, it causes scale formation on heat transfer surfaces.
The solubility of hardness salts decreases with the increase in temperature which results in deposition of salts in precipitate form on heat transfer surface
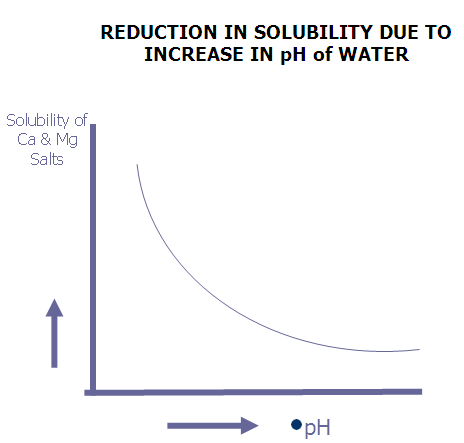
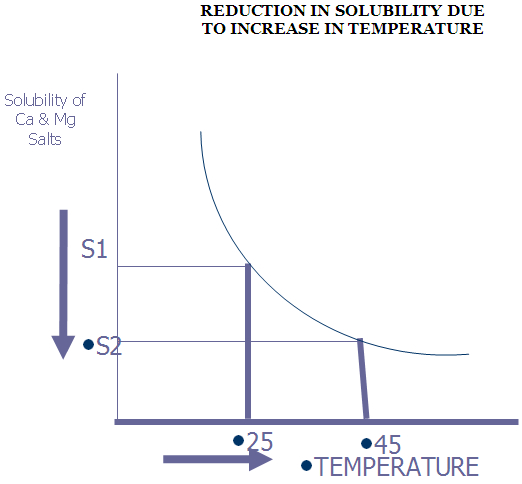
The solubility of hardness salts also decreases with the increase in pH which results in deposition of salts in precipitate form on heat transfer surface
Concerns of water usage in industrial applications
- Scaling 90-92 %
- Corrosion 4-5 %
- Algae 2-3 %